Description
Why is it necessary to measure the total O2 and CO2 content in the beer?

With the development of production technology and customers’ growing tasting ability, people become more sensitive about changes of beer appearance, aroma, flavor and wet disposition etc. People also come to realize increasingly the importance in controlling the total O2 and CO2 content in beer.
CO2 is a key component in forming the flavor of beer. The content of CO2 plays an important role in both the beer taste and beer filling process. A low content of CO2 affects the beer’s zest and foaming property while a high content will result in beer overflow during filling, thus affecting beer loss and foam making.
The total O2 content should be controlled precisely. An excessive O2 content will easily lead to an odor similar to the oxidation of fat, which affects the beer’s refreshing and mellow taste, and increases the after bitterness of beer. Excessive presence of O2 will also regenerate diacetyl which has been reduced previously, causing the beer to become “green and astringency” while oxidizing some of the flavor substances, thus deteriorating the quality of beer. As a result, it is necessary to measure the total O2 and CO2 in beer.
The World’s Leading Optical Probe

State–of–the–art TPO Analyzer
The CanNeed Total Package Oxygen Analyzer adopts the world’s most leading optical sensor, which is quick and exact in sensing and responding, and requires only a few samples to finish the test. It is also featured with personalized design, push button startup, automatic puncturing and auto measuring.
Suit your needs best
CanNeed can meet your needs and provide you with the most leading instrument for either the production site or the lab. The instrument is not affected by other dissolved gases and ensures the precision and stability of measurement.
Measuring both TPO & CO2
Push button startup, directly displaying the total content of O2 and CO2 in the LCD screen, clear display of test data of the dissolved O2, bottle neck space and CO2 content. No need of complicated calculation.
TPO Measuring Method – Principle of fluorescence measuring dissolved O2
When the atoms or molecules of matter absorb photon energy and become excited, and then return to the ground state from the lowest vibration level of the excited state. They will emit light of the same wavelength or of a longer absorption wavelength. This light is called fluorescence.
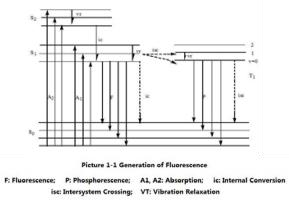
The Fluorescent Dissolved Oxygen Analyzer is based on the physical principle that certain substances quench with active fluorescence. The fluorescent matter in the front part of the sensor is made of special platinum porphyrin combined with polyester foil that allows gas through. The matter is coated with a layer of blackish light blocking material to avoid the interference of sunlight and other fluorescent substances in water.
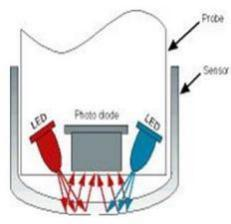
In order to use fluorescence to measure the dissolved Oxygen in the liquid, must ensure O2 get in contact with the fluorescent substance, and exert impact on the fluorescence release. In the optical O2 sensor, O2 penetrates the permeable layer, and then spreads to the matrix where the fluorescent molecules (dyes) are located. If there is no O2 present, most of the absorbed energy will be released as fluorescence. In the presence of O2, O2 comes into contact with dyes and absorb energy, thus not releasing fluorescence.
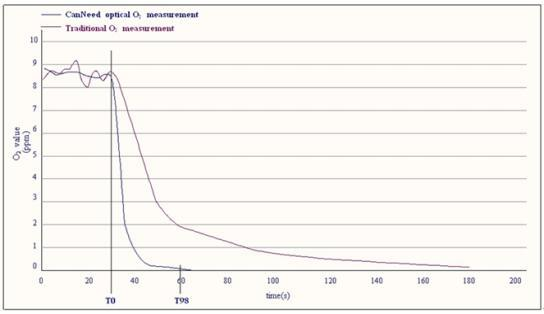
CanNeed-TPO-200 Contrast of response time and measurement principle with that of traditional method
The finding of O2 optical sensing was announced by Kautsky in 1939 and he demonstrated oxygen can dynamically suppress the fluorescence of lamps (decrease the quantum yield). It was also reported that the theory had been applied in many fields, such as monitoring aquatic organism in waste water, blood gas analysis and monitoring the culture of cells. ASTM (American Society for Testing and Materials) now approves this theory for testing oxygen in water. Compared with the traditional method of examining oxygen, which adopts the electrochemical sensor, the fluorescence technique is featured with absence of oxygen consumption, fast response, no need of electrolyte and minimal maintenance.
The traditional technique of measuring dissolved oxygen is based on the polarographic method. The dissolved oxygen membrane is filled with an alkaline electrolyte. Testing signals will be generated when oxygen diffuses through the membrane and reacts in the electrodes. Guess what: What will occur if the PH value of the electrolyte changes? Not only the measure performance, but also the dissolved oxygen signal will change. In presence of acid gases such as CO2, such hypothesis will be true. The acid gases will change the PH value of the alkaline electrolyte and result in the offset measurement value.
Response Time Curves: